The CRF2 Cleanroom Fogger uses 10 ultrasonic devices to produce 0.26 cubic meters per minute with 57 ml of fog density to provide 7-8 feet of visual airflow in fume hoods, bio-safety cabinets, glove boxes; while the CRF4 Cleanroom Fogger uses 35 ultrasonic devices to produce 1.25 cubic meters fog per minute with 170ml fog density to provide 10-15 feet visual airflow in small cleanrooms, ISO suites and sterile rooms. Fog is used in smoke studies to visualize airflow patterns and turbulence in Pharmaceutical ISO suites, sterile rooms, medical rooms and semiconductor clean rooms; providing compliance to USP 797 Pharmaceutical In-situ Airflow Analysis, Airflow Recovery Tests in ISO 14644-3, Visualization Tests for ISO 14644-3 Annex B7, NSF 49 for Bio-Safety Cabinets and Federal Standards 209E for semiconductor clean rooms.
Portable DI Water Fogger
Description
CRF2 Cleanroom Fogger and CRF4 Cleanroom Fogger! Which is best for your Application?
Cleanroom Fogger, DI Water producing 9cfm of Pure Fog – Request a Quote
Cleanroom Fogger, CRF-2 Features – Click here for Cleanroom Fogger Video

- 9 transducers converting DI Water or WFI water directly to 9cfm of of pure fog for 60 minutes
- Quick refill of Di Water or WFI water for continuous fog use, Easy to Fill Water Port and Water Level Indicator on left side of fogger
- Cleanroom Fogger Handle for easy carry convenience
- Polypropylene white enclosure provides a clean and particulate free enclosure, no finger prints, less weight
- Transducer life is 5000 hours
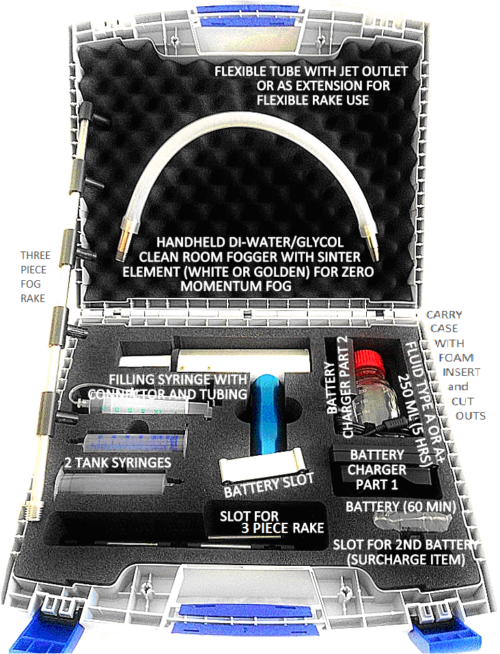
- Fog Curtain Wand, optional, plugs into end of Fog Hose to create a wide fog wall, while reducing the fog velocity and maintain a constant 9cfm of fog output
- 2″ Diameter (5.08 cm) hose that telescopes from 28 inches to 82 inches
- Fog Tube and 120VAC Power Supply (or 220VAC) is included, 15 foot power cable
- Easy to use, quick operation set up, instant On/Off fogging operation
- Rolling Carry Case, optional, for carry and storage of Clean room Fogger
- Remote Power Cable with ON/OFF switch to operate fogger behind closed walls or in a barrier isolator
- Paperless operation – instructions and applications on the right side of the cleanroom fogger

Cleanroom Fogger, Pharmaceutical Suites, Semiconductor Clean Rooms
- Supports Recovery Test evaluation, 100:1 recovery time as defined in ISO 14644-3
- Supports airflow visualization tests for ISO 14644-3 ANNEX B7
- Supports airflow visualization analysis for Pharmaceutical USP 797 Guidelines
- Supports airflow visualization tests for NSF 49 National Safety Foundation
- Supports airflow visualization tests for the future USP 800 Hazardous Drug Compounding
- Supports airflow visualization tests for Semiconductor Semi-Standards Guidelines
- Air flow balance
- Leak detection of exhaust ducts
- Barrier Isolator and laminar flow tests
- Wet bench exhaust tests
- Personal safety exhaust tests
- Chemical process equipment, exhaust ventilation tests
- Pressure balancing between rooms and spaces
- Visualization of airflow patterns and turbulence
Specifications, Clean Room Fogger– Request a Quote
(Details)
|
GENERAL INFORMATION
The CleanRoom Fogger is used in smoke studies as a smoke generator. It is a significant improvement over other Di Water Foggers by providing the highest volume output of all DI Water foggers sold today, reducing the weight by using a polypropylene enclosure rather then a costly SS enclosure. And Operation instructions are located on the right panel, so there is no paper manuals used in the pharmaceutical ISO 9 suites, laboratory or clean room.
Theory of Operation: Ultrasonic cavitation and DI water or Pharmaceutical WFI Water. Use of any other liquids or chemicals will void the warranty.
The transducer life is ~5,000 hours. To protect the transducers from damage there is a level sensor that will interrupt the input voltage to the transducer module, should the water level drop to a low level. This ensures long life and reliability.
Notes:
- The fog generated by this device contains microscopic droplets of DI water. AVOID USING IN IMMEDIATE VICINITY OF ELECTRICAL APPLIANCES, WATER SENSITIVE PRODUCTS AND EQUIPMENT.
- The fan will operate with no water in the reservoir with the power switch in the ON position. This will aid in drying when the chamber is drained.
- To increase drain plug tension, remove the plug, rotate clockwise (CW) the front latch while holding the rear metal disk.
- The CRF-2 is intended to be used on a flat surface, on its feet. Tipping the fogger with water in the reservoir will damage the fogger. DO NOT OVERFILL OR TIP THE FOGGER.
Which Clean Room Fogger or Smoke Generator Is Best For My Applications?
* Use Hand Gloves and Face Shield when filling LN2 |
16 Meg ohm DI water is standard |
Fogger Technology
The three types of foggers manufactured for use in the semiconductor and pharmaceutical industry are described below.
Ultrapure LN2 Fogger: This type of smoke generator or clean room fogger provides the highest volume, density and purity of fog. Purity is created by bringing the water to a high temperature, creating a vapor, while simultaneously using gravity to remove the residual mass from the vapor. This process removes any bacterial agents and residual particulate matter from the vapor. The pure vapor is then passed over an LN2 bath, which naturally boils at room temperature. The water molecules bond with nitrogen molecules, creating a nominal 3um fog droplet. The volume of water and nitrogen molecules that combine is extremely high in quantity, creating a dense, high volume, ultrapure fog output with exit temperatures of about 78 degrees F with an exit pressure of less than 0.5 lbs, so as not to disturb the surrounding airflow. The fog is ultrapure leaving minimal, if any, trace particles behind. It evaporates to its gaseous hydrogen, oxygen and nitrogen components, which are natural to the Cleanroom environment. The high density of the fog increases the duration and travel distance of the fog. This fogger can be used in a Class 1 – 10,000 cleanroom environments of pharmaceutical and semiconductor facilities; such as sterile rooms, hospital rooms, medical rooms and cleanrooms.
DI Water Fogger: This type of fogger has less fog density (less capability to visualize airflow) than the UltraPure Fogger described above, but more density than the CO2 fogger described below. The DI water fog is generated by atomizing DI water into water droplets, which are nominally 3-10um in size. The water droplets may contain residual particulate matter remaining in the DI water, but this would be very trace amounts. If the facility manager operates a class 10 to Class 10000 Clean room, the use of a DI Water Fogger poses no problem. However, Cleanroom Engineers who manage facilities operating at Class 1 to Class 10 performance may desire to use an ultrapure fogger. Although some DI Water foggers are described as ultrapure, unless the DI water is vaporized to remove bacterial agents and residual particulate matter, the fog is not ultrapure. The 3-5lb output pressure of a DI water fogger also distorts the airflow patterns, thus adding to the turbulence. The temperature output is typically less than the surrounding room temperature, thus a fog generated from the atomized water droplets will sink momentarily in a typical 70 degree room temperature.
CO2 Fogger: This type of smoke generator or CO2 Fogger is designed for low volume, non-process critical applications such as bench airflow testing. The fog is created using CO2 ice as the fogging agent. The fog contains elements of the CO2 and the user must determine if the residual CO2 components are acceptable in a process environment operating Class 100 to Class 10,000. The 3-5lb output pressure of a CO2 fogger also distorts the airflow patterns, thus adding to the turbulence. The output starts at about 3cfm and slowly decreases to 0 CFM in about 10 – 12 minutes.
Smoke Sticks
Smoke Sticks are used in some Pharmaceutical Clean Rooms around the world. Below is a discussion on the use of smoke sticks used to visualize airflow and turbulence?
A smoke stick is often used visualize airflow turbulence, but smoke sticks are filled with particulates and chemicals. Smoke is created using chemical reactions; thus the smoke is SPUTTERING (sputter) or popping out of the smoke stick in a non-consistent pattern with velocity, but little volume. It is a particle smoke, compared to a visible, pure water based fog, thus smoke sticks are a contaminating smoke. The smoke stick generates an inconsistent flow or pattern of smoke, but it is low cost, which is why some managers allow use of smoke sticks in their Pharmaceutical clean rooms.
Compare a smoke stick to a Clean Room Fogger or an UltraPure LN2 fogger, both which produce a constant volume of fog with a consistent fog output and pure fog. Di Water foggers produce a consistent flow of visible water vapor, which enters the airflow to visualize the airflow patterns and turbulence, then begins to evaporate, returning back to the hydrogen, oxygen and nitrogen components that we breathe. No particulate contamination, no chemical contamination. Water based foggers produce a constant volume of fog at a constant rate, which provides consistent visualization of airflow patterns and turbulence. The Smoke Stick has to be waved around to see what kind of airflow pattern there is, while a Di Water fogger is simply placed in position and produces a flow of fog that can be directed 360 degrees to easily describe the airflow patterns and turbulence. In addition, tubes are now available to create “fog curtains”, or a wall of fog, which smoke sticks can not produce.
How many smoke sticks are used per smoke cycle? How much labor is needed to clean up after smoke stick use. Do you need to Clean all the walls where the smoke stick was used. How did the chemical particulates and particles affect the process area? These are critical questions for a pharmaceutical manager. Did the contaminating particles and chemicals get into the drug process?
How much labor is used to cleanup after smoke stick use and if the cleanup did not get every chemical particle, then some smoke chemical material is added to the Pharma process or trapped in a filter somewhere, until it escapes into the Pharma process. That is a quality control issue for that company using smoke sticks.
The low labor cost of using smoke sticks is the reason facility managers may use smoke sticks, but are the chemical and particulate effects to the pharma process being analyzed? Non-contaminating fog does not emit particulates, requires less labor and does not contribute any unwanted chemicals to the Pharma process. A Di Water Fogger provides these advantages in fog volume, fog consistency and fog purity, which easily outweighs the low cost of smoke sticks, the high cost of labor for cleanup and the detrimental affects to quality control!
Smoke Sticks – quality side of the drug product: The smoke chemicals are not of the same chemistry as the drug product, thus smoke chemicals and particulates could migrate into the drug process. There is no guarantee the cleaning process removed all the unwanted particulates and chemicals, from for example, a glove box or isolation box. The chemicals and particulates eventually migrate to the air filter system, which is not 100% effective. If this is the case, the quality and purity of the drug process is affected. Drug quality is the basis of product credibility, which is a valuable asset in customer relations.
Smoke Sticks – labor side of the drug product: The smoke is generated by a chemical reaction, which causes the smoke to sputter into the environment. The smoke is inconsistent in volume, thus the smoke stick is unpredictable for airflow visualization. The chemicals migrate to equipment and walls, which then must be cleaned, and requires an added labor cost. The use of Smoke sticks generates an inefficient smoke, not a consistent fog.
Glycol Foggers – Glycol foggers use water combined with a glycol liquid, which has a long strand, glycol molecule. Glycol produces produces excellent visible fog; but the long strand molecules degrade HEPA filters by choking the filter material. This reduces airflow efficiency, and eventually an odor within the filter and airflow occurs. In most cleanrooms, glycol is considered a contaminate, as the glycol residue needs to be wiped clean, requiring more labor and manpower. In a pharmaceutical process, how does the facility manager clean 100% of the glycol residue from the clean room? How much of the glycol residue enters into drug process?
A Di Water Fogger produces a water (H2O) droplet that evaporates back into hydrogen and oxygen, the air we breathe. No clean up is required, at all. No additional time delays and clean up labor is not required. The fog is consistent in volume and constant in output to describe the airflow patterns and turbulence. These are equipment, quality and application concerns to consider when the need for airflow visualization is considered.